- Dec 4, 2025
The Role of CTNNB1 Gene Mutations in Neurodevelopment and Neural Function
- Faisal Jahangiri
- ionm, neurophysiology, Blogs, neuroscience
- 0 comments
Imagine receiving the joyful news that you are expecting your first child. The day finally arrives, and your baby is born, healthy, beautiful, and full of promise. As the months go by, your home is filled with laughter and love. But soon, you notice that something isn’t quite right with your child’s development. When he starts learning to walk, every small step seems to be a struggle. Concern turns into worry, and after two long years of medical appointments, testing, and unsuccessful treatments, you finally receive a diagnosis, CTNNB1-associated neurodevelopmental disorder.
This is the story of a family friend, a reminder that behind every rare genetic condition lies a family’s journey of uncertainty, strength, and hope.
CTNNB1 gene encodes β-catenin [1], a key protein involved in adherents junctions, where it links cadherins to the actin cytoskeleton to maintain proper cell-to-cell communication. This protein plays an essential role during embryonic development, particularly in regulating cell adhesion and signaling pathways critical for brain formation [2]. Mutations in the CTNNB1 gene disrupt these processes, leading to cognitive impairments and developmental delays. In essence, when a gene responsible for maintaining communication between brain cells malfunctions, the entire process of neural development can be profoundly affected [3].
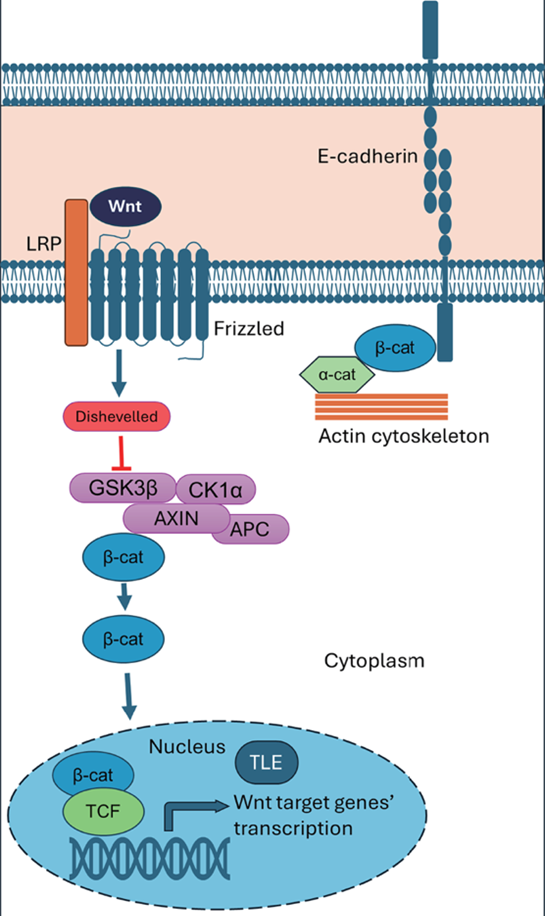
Figure 1. Represents the major function of Beta-catenin in bilaterian organism.
B-catenin serves both signaling and structural roles within the cell. In signal transduction, when Wnt ligands (protein that plays a role in signaling) are present, the β-catenin degradation complex is inhibited by Disheveled (Dvl) [4]. This inhibition allows β-catenin to accumulate in the cytoplasm and translocate to the nucleus, where it binds to TCF (T-cell factor) transcription factors and displaces the repressor TLE (transducing-like enhancer). This interaction initiates transcription of canonical Wnt target genes, which are crucial for cell proliferation and differentiation. [5]
CTNNB1 syndrome is a neurodevelopmental disorder characterized by microcephaly (a birth defect in which a baby’s head is significantly smaller than expected), facial dysmorphism (distinct facial structural differences), axial hypotonia (reduced muscle tone in the central body), and peripheral spasticity (increased muscle stiffness in the limbs). Despite these neurological and physical symptoms, brain imaging such as MRI often reveals no structural abnormalities [3].
CTNNB1 syndrome is often misdiagnosed as cerebral palsy (a group of movement disorders that affects posture, balance and coordination) because the two conditions share several overlapping symptoms, such as developmental delays, motor difficulties, and muscle tone abnormalities [1]. However, there is a key difference between the two: in cerebral palsy, brain imaging such as MRI typically reveals structural abnormalities or lesions in the brain, reflecting early injury to developing neural tissue. In contrast, individuals with CTNNB1 syndrome usually show no detectable brain abnormalities on MRI, even though they experience significant motor and cognitive impairments [6].
Current studies suggest that CTNNB1 syndrome affects males and females equally, with no known gender bias in its occurrence. This genetic condition is extremely rare, as of early 2024, only about 430 individuals worldwide have been diagnosed with CTNNB1-associated neurodevelopmental disorder. Because of its rarity, ongoing research and global patient registries are essential to better understand symptoms, genetic variations, and potential therapeutic strategies [3].
Unfortunately, CTNNB1 syndrome results from a de novo mutation, meaning it occurs spontaneously and is not inherited from the parents. Because of this, there is currently no way to prevent the disorder or directly treat the condition. However, available treatments focus on managing the underlying symptoms and associated disorders, such as developmental delays, motor impairments, and speech difficulties [3].
Diagnosis is primarily based on characteristic clinical features observed during medical evaluation. Once these features raise suspicion, the physician typically discusses molecular genetic testing with the family to confirm the presence of a CTNNB1 gene mutation. Early diagnosis allows for better planning of supportive therapies and educational interventions to improve quality of life.
Because there is still limited information available about CTNNB1 syndrome, there are numerous clinical and research directions currently being explored. Organizations such as the CTNNB1 Connect & Cure Foundation and the CTNNB1 Foundation, along with researchers and clinicians worldwide, are dedicated to finding effective treatments and, ultimately, a cure for CTNNB1 syndrome. Since 2019, these groups have united experts from diverse scientific fields and invested in multiple research initiatives aimed at advancing therapeutic discovery [6].
Simons Searchlight has also become an important global platform by remotely collecting high-quality, standardized data on the natural history of CTNNB1 syndrome and making it accessible to researchers. Efforts such as genotype–phenotype correlation studies and biochemical characterization of CTNNB1 mutations are helping scientists understand how specific genetic variants lead to distinct clinical symptoms. Recent advancements include the generation of induced pluripotent stem cells (iPSCs) from patient samples and the development of preclinical mouse models, both of which provide valuable insight into the molecular and functional consequences of CTNNB1 haploinsufficiency. Multiple therapeutic strategies are currently in development, including small-molecule drugs, RNA- and DNA-based therapies, and an AAV9 gene replacement therapy, which entered the manufacturing phase in November 2023 [7].
The vision of the CTNNB1 research community is to establish several effective therapeutic options in the near future, giving hope to families and patients affected by this rare genetic disorder.
CTNNB1 syndrome is a rare neurodevelopmental disorder resulting from de novo mutations in the CTNNB1 gene, which encodes β‑catenin a protein critical for cell signaling, adhesion, and embryonic brain development. The condition affects males and females equally and is typically characterized by microcephaly, global developmental delay, motor impairment, axial hypotonia, and distinct facial features, though MRI findings usually reveal no structural brain abnormalities. Because its clinical presentation often overlaps with cerebral palsy, misdiagnosis is frequent. With only about 430 documented cases worldwide, there is currently no cure; management focuses on addressing developmental and motor challenges through supportive therapies and multidisciplinary care.
Global collaborations spearheaded by the CTNNB1 Connect & Cure Foundation, the CTNNB1 Foundation, and Simons Searchlight are driving cutting-edge research into genotype–phenotype correlations, the development of iPSC and mouse models, and the advancement of emerging gene replacement strategies, including an AAV9-based therapy now in production [8].
Many neurodevelopmental disorders remain underrecognized and underdiscussed, often because of their rarity and complex genetic origins. This reality underscores the urgent need for clinicians and scientists to cultivate a deep understanding of human physiology, biology, and chemistry. Such knowledge is essential not only to advance diagnostic accuracy and guide innovative research, but also to ensure that patients affected by these rare conditions receive the comprehensive and compassionate care they deserve.

About the Author:
My name is Leslie Gonzalez, and I am currently enrolled at the University of Texas at Dallas, where I am pursuing a bachelor’s degree in Neuroscience. My long-term goal is to become a gynecologist, combining my passion for medicine with my desire to support women’s health. Outside of academics, I enjoy reading and spending quality time with my family. I also volunteer with my local fire department, an experience that has strengthened my ability to work under pressure and remain calm in stressful situations. Through my dedication and hard work, I was honored to receive the Rookie of the Year award. This role has deepened my commitment to serving my community and helping others during critical moments.
References:
Administrator. (2025, October 29). Dallas Cerebral Palsy Lawyers: National Birth Injury Law. National Birth Injury Lawyers. https://www.nationalbirthinjurylaw.com/dallas-cerebral-palsy-lawyers?NBIL-BI-DAL&campaign=18023056452&content=618836757317&keyword=cerebral+palsy&gad_source=1&gad_campaignid=18023056452&gbraid=0AAAAADpovSltM92Uk079C2DrgOr-3TATz&gclid=CjwKCAiAt8bIBhBpEiwAzH1w6ZLL4lOOyhI3Ye1493xxmP2qdTal4VY4AoX0wmebe4XRPjalbN9ndxoCzqYQAvD_BwE
Https://www.sciencedirect.com/science/article/abs/pii/s1051200421000968 | request PDF. (n.d.-b).
CTNNB1 syndrome - symptoms, causes, treatment: Nord. National Organization for Rare Disorders. (2024, July 26). https://rarediseases.org/rare-diseases/ctnnb1-syndrome/
Mbogo, I., Kawano, C., Nakamura, R., Tsuchiya, Y., Villar-Briones, A., Hirao, Y., Yasuoka, Y., Hayakawa, E., Tomii, K., & Watanabe, H. (2024, December 2). A transphyletic study of metazoan β-catenin protein complexes - zoological letters. BioMed Central. https://zoologicalletters.biomedcentral.com/articles/10.1186/s40851-024-00243-y.
Cell adhesion - an overview | sciencedirect topics. (n.d.-a). https://www.sciencedirect.com/topics/medicine-and-dentistry/cell-adhesion.
Gene therapy. CTNNB1 Foundation. (n.d.). https://ctnnb1-foundation.org/gene-therapy.
Miroševič, Š., Khandelwal, S., Amerson, E., Parks, E., Parks, M., Cochran, L., González Hernández, A., Ferraro, M., Lisowski, L., Perez-Iturralde, A., Chung, W., Jacob, M. H., Žakelj, N., Lainšček, D., Forstnerič, V., Sušjan, P., Maruna, M., Jerala, R., & Osredkar, D. (2025, February 12). Paving the way toward treatment solutions for CTNNB1 syndrome: A patient organization perspective. Therapeutic advances in rare disease. https://pmc.ncbi.nlm.nih.gov/articles/PMC11822810/